The Genetic Frontier: Can Africa Seize the Cell and Gene Therapy Opportunity?
Cell and gene therapies (CGTs) are no longer science fiction. Globally, we're seeing a wave of innovative therapies that can correct or replace faulty genes, offering the possibility of cures for genetic diseases once thought to be lifelong or fatal. But with price tags often north of $1 million per treatment and the incredible sophistication required to administer these therapies, questions about access, affordability, and readiness should be front and center. Yet in Sub-Saharan Africa, this conversation hasn’t fully taken shape yet.
A Global Surge – But Not Global Access
In high‑income markets like the US, EU, and Japan, more than 40 CGTs have received regulatory approval and have already entered clinical practice. These CGTs treat a range of conditions — from CAR-Ts, which involve reprogramming a patient’s own immune cells (specifically T cells) to recognize and attack certain blood cancers, to gene therapies for rare neurological conditions. However, while clinical trials and even early CAR‑T programs are underway in some African countries (e.g., South Africa & Egypt), no CGT has yet reached routine clinical use in Africa.
There are upwards of 500 more CGTs in the pipeline, yet the global distribution of trials unveils a stark imbalance in where innovation is happening and where it isn’t. According to clinicaltrials.gov, the largest database of clinical research in the world, as of mid-2025, over 450 interventional gene therapy trials were actively recruiting worldwide — but only five of those are in Africa. Egypt hosts four of them, and South Africa just one. This stands in stark contrast to countries like the United States (222), China (156), and the EU4+UK region (157), underscoring how unevenly the CGT revolution is unfolding. If innovation in gene therapies continues to bypass Africa, what does that mean for their future on the continent and for the patients who need them most?
One of the most high-profile breakthroughs in this space is the arrival of gene therapies for sickle cell disease, a condition that has caused immense suffering for generations. Treatments like Casgevy and Lyfgenia have been approved in the US, UK, EU, and UAE, with price tags that can be over $2 million per patient. Yet none are available in Sub-Saharan Africa, where the disease is most concentrated. This absence is not simply the result of a lack of will. These therapies are incredibly complex to deliver, and many African health systems are not yet set up to support them. From limited diagnostic capacity to the absence of mature and sustainable reimbursement pathways, the barriers are real. But if we do not begin addressing them now, we risk a future where the most advanced innovations in medicine continue to bypass the places that need them most.
Why Africa Is Uniquely Positioned
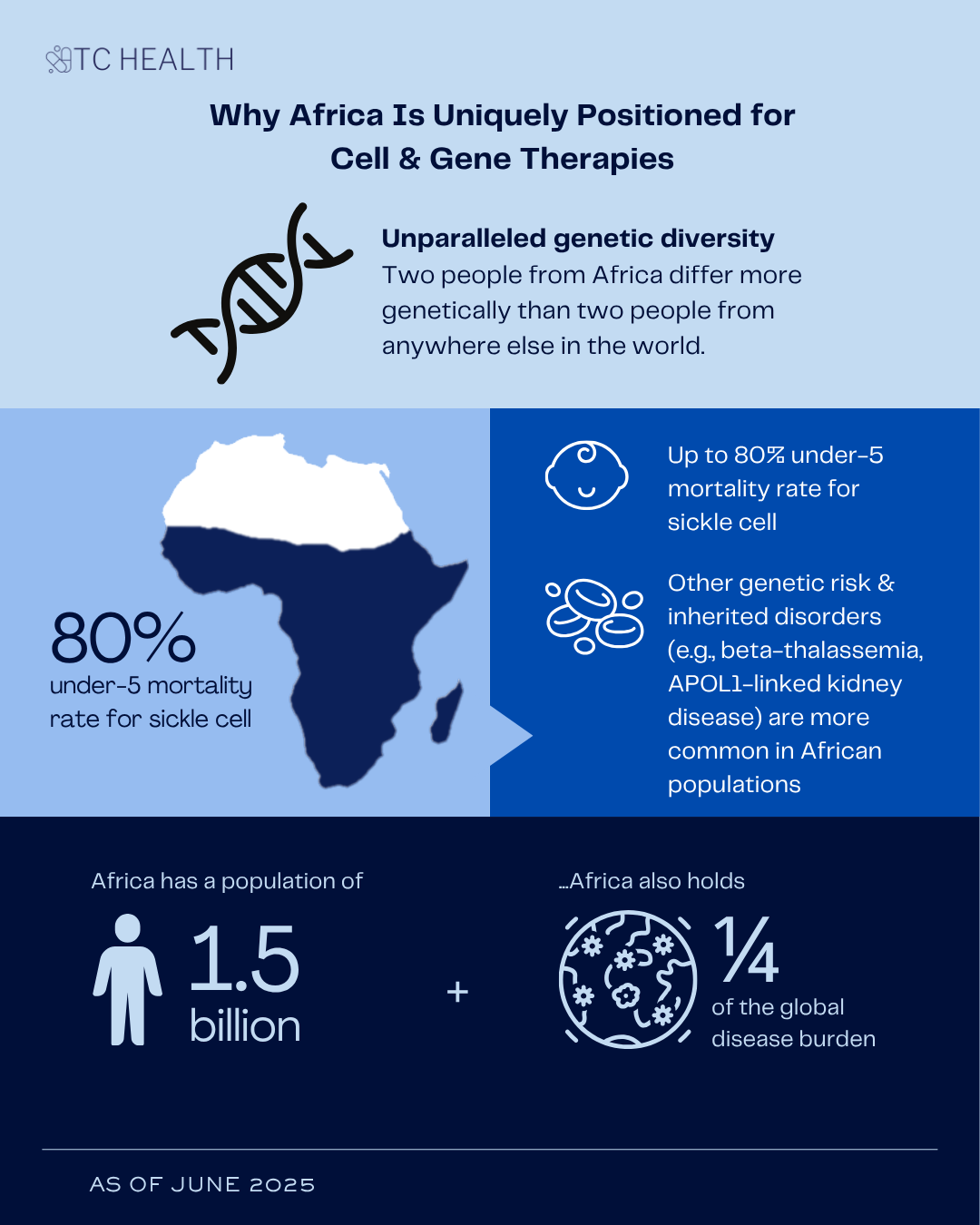
While Africa may not yet be administering CGTs, it has something the rest of the world doesn’t: unparalleled genetic diversity. Two people from Africa differ more genetically than two people from anywhere else in the world. African genomes hold the richest variety of genetic variants anywhere on the planet. This makes the continent not just a potential beneficiary of gene therapy but a vital contributor to its development.
The scale of the health burden further strengthens the case. Sickle cell disease, one of the world’s most common and devastating inherited conditions, is particularly concentrated in Africa. About 80% of sickle cell disease cases occur in Sub-Saharan Africa, and the mortality rate for children under 5 years of age can be as high as 80%. Other inherited blood disorders like G6PD deficiency and beta-thalassemia are also widespread and underdiagnosed. APOL1-linked kidney disease and emerging gene variants tied to diabetes are being found disproportionately in African populations. In short, Africa has both the need and the biological basis to lead.
Add to that a population of 1.5 billion as well as a quarter of the global disease burden and the potential for genomic advancements becomes even more compelling. So, the question isn't whether CGTs are relevant to Africa, it's when, and how, they will arrive.
Is the Continent Ready?
Despite the opportunity, the readiness gap remains a real problem to tackle. To unlock access, Africa must overcome several layers of complexity, many of which remain unresolved, even in high-income settings.
How Does Africa Pay for Million-Dollar Therapies?
In the US, where the payer landscape is highly fragmented, value-based reimbursement for CGTs — a healthcare payment model where manufacturers are paid based on how well a treatment works, rather than just selling the product at a fixed price — has seen limited uptake. These one-time therapies often cost over $2 million, posing a major risk to insurers if benefits don’t last. While pay-for-performance models were designed to share this risk, pilots have struggled to scale, largely because patients often switch insurance plans every few years and challenges exist in tracking long-term clinical outcomes. As a result, most insurers still rely on upfront payments amid significant uncertainty around the durability of these treatments.
And these challenges are not unique to the US; in Europe, several countries introduced value‑based payment pilots for CAR‑T therapies. Spain and Italy implemented staged, outcome‑linked payments when the first CAR-Ts launched, meaning they paid in instalments only if patient results were positive. Germany, one of the continent’s largest pharmaceutical markets, on the other hand, tested outcomes‑based rebates (partial refunds of payments already made) during an initial period of free pricing, but efforts to expand beyond these pilot agreements stalled at the pre‑reimbursement stage overseen by the G‑BA (Germany’s authority for evaluating and approving drug reimbursement).
This shows that even well‑resourced health systems face real challenges rolling out value‑based payment models for advanced therapies — challenges that are only magnified in settings like Africa's. Many African countries face similar fragmentation to the US, with out-of-pocket payments, underfunded public systems, and nascent insurance markets. In these settings, installment-based models may be more feasible than performance-based ones, which require robust data systems and long-term follow-up.
How Do We Create More CGT Centers of Excellence in Africa?
CGTs involve complex procedures, such as collecting a patient’s cells, modifying them in a lab or factory, and safely re-infusing them back into the body. Patients must also be closely monitored for safety and potential adverse events, often through specialized pharmacovigilance protocols. Such treatments must be delivered in facilities accredited under rigorous standards, like JACIE in Europe or FACT in the US. These accreditation systems require strict quality management, specialized infrastructure, and well-trained multidisciplinary teams, meaning only a limited number of institutions are equipped to meet the criteria. As a result, even within Europe, access remains uneven. Countries like Ireland, Hungary, and Greece have only a handful of JACIE-accredited centers, while others such as the UK, Germany, and France have 20 or more.
So, how can African hospitals get there? This will require significant investment in human capital, including the training and retention of multidisciplinary teams such as haematologists and transplant physicians, as well as improvements to quality management systems, infrastructure, and safety protocols. The good news is that some African hospitals are already making progress. South Africa is currently the only country on the continent with JACIE-accredited centers, with three now in place. This shows that the groundwork for safely delivering advanced CGTs on the continent is already underway.
How Can Africa Build a Supply Chain That Delivers on Time?
CGTs need to be made and delivered under significantly tight timelines and strict temperature controls, which makes fast and reliable logistics essential. That’s a big challenge in many parts of Africa where infrastructure is limited. But even in high-income countries, manufacturing and distribution remain difficult. Experts still point to major hurdles; from not having enough treatment centers, to relying on a single supplier for key materials, to the fact that every patient’s cells are different, which makes standardizing the process difficult. And while automation is helping, much of the work still depends on clean rooms and manual labour. To mitigate these challenges, pharma companies have invested heavily to improve manufacturing. Pfizer alone has spent over $800 million in gene therapy manufacturing and global investments in the sector are expected to reach nearly $12 billion by 2028.
For Africa, one of the most practical solutions is to build manufacturing capacity closer to home; that is, on the continent or in nearby regions like Southern Europe or the Middle East. Yet with limited local production of even basic drug ingredients, this will require major investment in infrastructure, skills, and regulation. Still, it is a critical step to make CGTs truly accessible.
RELATED: Empowering Independence: Advancing Local API & Monoclonal Antibody Production in Africa
Additionally, most cell therapies available today are autologous, meaning they are made from each individual patient's own cells and require highly personalized manufacturing and handling. But new allogeneic, or “off-the-shelf,” approaches are beginning to emerge. These therapies are made from donor cells and can be stored, transported, and delivered more like traditional injectable drugs. Their standardized production and simpler logistics make them a more promising option for low-resource settings like many across Africa.
Is Africa’s Diagnostic System Ready for the Genomic Era?
To identify who can benefit from CGTs, we need much better access to genetic testing. At the moment, most of this testing still takes place outside the continent, which leads to longer turnaround times, higher costs, and logistical challenges. In South Africa, for example, a recent study found that over 90 percent of rare disease patients went undiagnosed when only small gene panels were used. This highlights the need for broader access to technologies like exome sequencing.
RELATED: Nigeria's Diagnostic Industry: Battling the Double Burden of Disease
While access remains limited in many African countries, efforts are underway to expand local capacity through regional labs, training programs, and new partnerships. Across the continent, initiatives like H3Africa have emphasized the lack of routine next-generation sequencing and the limited representation of African genomic data in global databases. H3Africa supports genomics research in Africa by building local infrastructure, training scientists, and generating data to understand how genetic and environmental factors influence health across African populations. Improving local sequencing is about bringing Africa's unparalleled genetic diversity into global datasets, which can lead to better, more inclusive therapies not just for African patients, but for everyone, everywhere.
The Bottom Line
The good news is that the building blocks are already coming together. Initiatives like H3Africa and 3MAG are helping to scale genomic research. At the African Centre of Excellence for Genomics of Infectious Diseases (ACEGID), Professor Christian Happi is pioneering the use of CRISPR-based diagnostics and gene editing tools tailored to African public health needs, pushing the boundaries of how local genetic data can drive innovation. Startups like Yemaachi are tapping into African genetic data to inform more targeted therapies for cancer. And institutions like the African Medical Centre of Excellence (AMCE) in Nigeria, which recently secured a $75 million research grant, are investing in the infrastructure needed to deliver and evaluate advanced treatments.
These are promising signals, but several critical questions remain to turn these signals into real progress. How can we reverse brain drain and build a pipeline of specialists equipped to deliver CGTs? How do we sustainably finance these treatments in health systems already strained by high out-of-pocket spending and low public investment? From a pricing perspective, could volume-based arguments help unlock more affordable access, particularly for gene therapies which are currently priced as rare disease drugs in high-income markets despite being far more common in Africa (i.e., sickle cell disease)? Answering these questions, and many more, will be key to unlocking CGT access on the continent.
Africa has a compelling case to be a leader in the era of genetic medicine. The continent holds the world's deepest genomic insights and carries some of the highest burdens of treatable inherited diseases. With the right investment, infrastructure, and innovation, Africa could leapfrog into a new frontier of care. But the window to prepare is now. We can’t expect — or afford — to wait decades for access to the latest therapies. The question is: are we ready to join the race?